
Charge is shown on the upper right portion. Symbol for the element is Na, but the symbol for the ion is Na 1+.Example: Sodium positive 1+ ion has 10 electrons and 11 protons.Example: Sodium has 11 electrons and 11 protons when it is neutral.Notice the charge of the ion is shown on the upper right portion of the symbol. For example, Cl is the element symbol, but the symbol for the ion is Cl 1. Know the difference between the symbol for the element compared to its ion.Example: Chlorine negative 1- ion has 18 electrons but 17 protons.Example: Chlorine has 17 electrons and 17 protons when it is neutral.The charge is determined by the difference in the number of protons and electrons in an atom.Number of protons remains the same when ions are formed.Nonmetals tend to gain electrons to form negative ions.Metals tend to lose electrons to form positive ions.Atoms tend to gain, lose or share electrons to get valence electron configuration similar to the noble gases (Group 18).Ion is an atom or group of atoms with a positive or negative charge.Group 18 (Noble Gases) elements have a "stable electron configuration".Example: Group 17 elements have 7 valence electrons.Example: Group 1 elements have 1 valence electron.Elements in the same Representative Group have similar chemical properties because they have the same number of valence electrons.Elements in same Representative Group have same # of valence electrons, except helium in Goup 18.Elements in same Period have same # of energy levels or shells.Hydrogen is the only element on the left side of the BOLD "staircase" boundary that is a nonmetal.Elements with boundaries on the staircase are the metalloids, except alumunum ("Al"). Look for the BOLD staircase in your Periodic Table as the "boundary between metals and nonmetals. All elements are classified as metals (left side), nonmetals (right side, but not including Group 18 elements) or metalloids.Transition Elements (also called Transition Metals) are Groups 3 to 12.Representative Elements are Groups 1,2, and 13 to 18.For example, all the elements in Period 5 have 5 energy shelss and their valence electrons are in the 5th energy shell (highest energy shell). Elements in the same Period have the same number of energy shells.Elements in a row are referred to as being in the same Period (7 Periods).Elements in a column are referred to being in the same Group (18 Groups).Periodic Law: There is repetition of chemical properties when elements are arranged by increasing atomic number.Periodic Table is organized by increasing atomic number (equal to number of protons).The number of unpaired electrons gives an indication of how an element combines with other elements. The Lewis Dot Diagram highlights the number of paired and unpaired electrons.3) Number of valence electrons ranges from 1 to 8 depending on the element.2) Show each valence electron one at a time using the "clock" method by placing the electrons at 12 3 6 and 9 oclock.Lewis Dot Diagrams show only the valence electrons of an element.Sometimes just referred to as Lewis Electron Dot Diagram.With Clean clear, easy-to-learn and easy-to-understand explanations for students.Finally, something new and different for Chemistry students. With Clean clear, easy-to-learn and easy-to-understand explanations for students.ģ0 Days of Practice Question Set for the New York State Chemistry Regents Exam, The Physical setting. Surviving Chemistry Regents Exam One Day At A Timeģ0 Days of Practice Question Set for the New York State Chemistry Regents Exam, The Physical setting. I am unaware of the original author and do not claim to be the original author. Based off of a handout I had my first year of teaching. Outline of the basic topics on Kinetics and Equilibrium. Outline of the basic topics on Chemical Bonding. Outline of the basic topics on Acids, Bases and Salts.
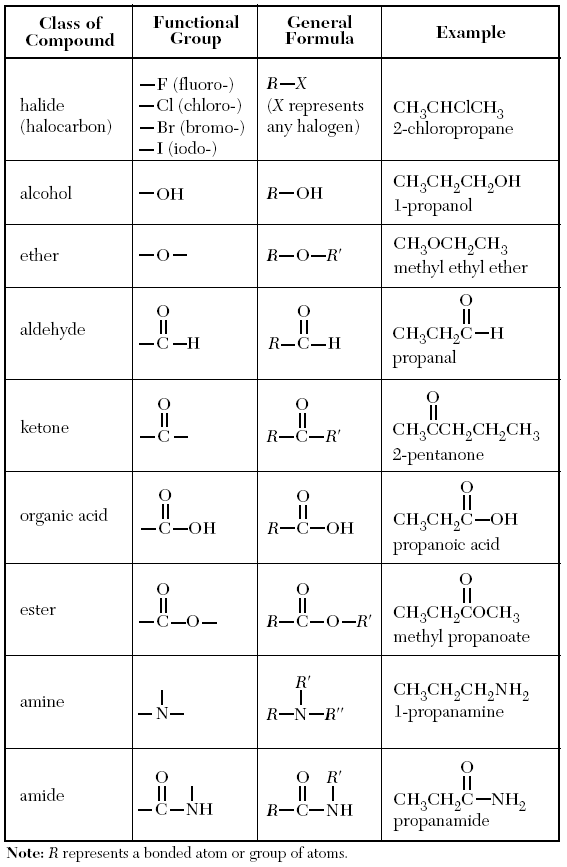
Outline of the basic topics on the Periodic Table. Outline of the basic topics on Atomic Structure and History. Multiple Choice Test for assessment and exam preparation 200 Ways to Pass the Chemistry Physical Setting Regents Exam


 0 kommentar(er)
0 kommentar(er)
